What Is The Chemical Makeup Of Fluoride
What is Sodium Fluoride?
Sodium fluoride is manufactured by the reaction of hydrofluoric acid with sodium carbonate or sodium hydroxide with the formula NaF.
The about cheap chemical bachelor for fluoridation is sodium fluorosilicate, formerly known as sodium silicofluoride . Sodium fluoride solutions are used with difficult h2o insoluble compounds of calcium and magnesium fluoride can form. Information technology is a dry chemical used in fluoridation of drinking h2o, it should exist manually weighed and added to the mixing tank.
| NaF | Sodium fluoride |
| Density | 2.56 g/cm³ |
| Molecular Weight/ Molar Mass | 41.98817 thousand/mol |
| Boiling Point | 1,695 °C |
| Melting Betoken | 993 °C |
| Chemical Formula | NaF |
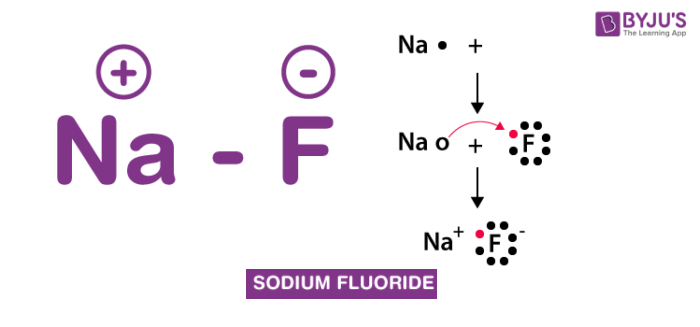
Sodium Fluoride Structure – NaF
Physical Backdrop of Sodium fluoride – NaF
| Odour | Odourless |
| Appearance | White crystals or powder |
| Complexity | 2 |
| Vapour Pressure | i mm Hg at 1971 ° F |
| Hydrogen Bond Acceptor | 1 |
| Solubility in water | 10 to 50 mg/mL at 73° F |
Chemical Backdrop of Sodium fluoride – NaF
-
- Sodium fluoride reacts with h2o forms hydrogen fluoride and sodium hydroxide. The chemic equation is equally below.
NaF + H2O → HF + NaOH
-
- Sodium fluoride reacts with chlorine undergoes deportation reaction forming sodium chloride and fluorine.
NaF + Cl2 → NaCl + F2
Uses of Sodium fluoride – NaF
- Sodium fluoride has bactericidal properties simply it is too toxic for use as a wound antiseptic.
- Systemic fluoride use during pregnancy has not been shown to prevent tooth decay in children
- Sodium fluoride is used as an insecticide.
- Used in the fluoridation of public water.
- The mono sodium table salt of Sodium fluoride 50-glutamic acrid; used in treatment of encephalopathies associated with liver diseases.
Frequently Asked Questions
What is sodium fluoride used for?
Sodium fluoride is used for the cavity prevention. This makes the teeth healthier and more resistant to acid and bacteria causing disuse. Used for the treatment of osteoporosis and otospongiosis in adults, its use is controversial and further studies are expected.
Where does fluoride come up from
Fluoride is a mineral that occurs in all natural h2o bodies worldwide. This dissolves into the groundwater like iron and calcium, which we rely on for our drinking water.
What is fluorides function in the torso?
Fluorine is necessary for our bones to be preserved and solidified, and prevents dental decay. Nevertheless, if it is ingested too often, it tin can reverse outcome causing rotting of the teeth, osteoporosis and damage to the kidney, bone, nerve and muscle as well.
When was h2o fluoridation introduced?
In 1945, Thou Rapids, Michigan, changed the water supply fluoride content to 1.0 ppm and thus became the first city to introduce water fluoridation in the world.
What are the benefits of water fluoridation?
Drinking fluoridated h2o keeps teeth make clean and decreases cavities in children and adults likewise known as tooth disuse by around 25 per cent. Through avoiding cavities, fluoridation of drinking h2o has been shown to relieve money both for households and for the U.s. health care system.
What Is The Chemical Makeup Of Fluoride,
Source: https://byjus.com/chemistry/sodium-fluoride/
Posted by: smithspoe1957.blogspot.com


0 Response to "What Is The Chemical Makeup Of Fluoride"
Post a Comment